Procedure
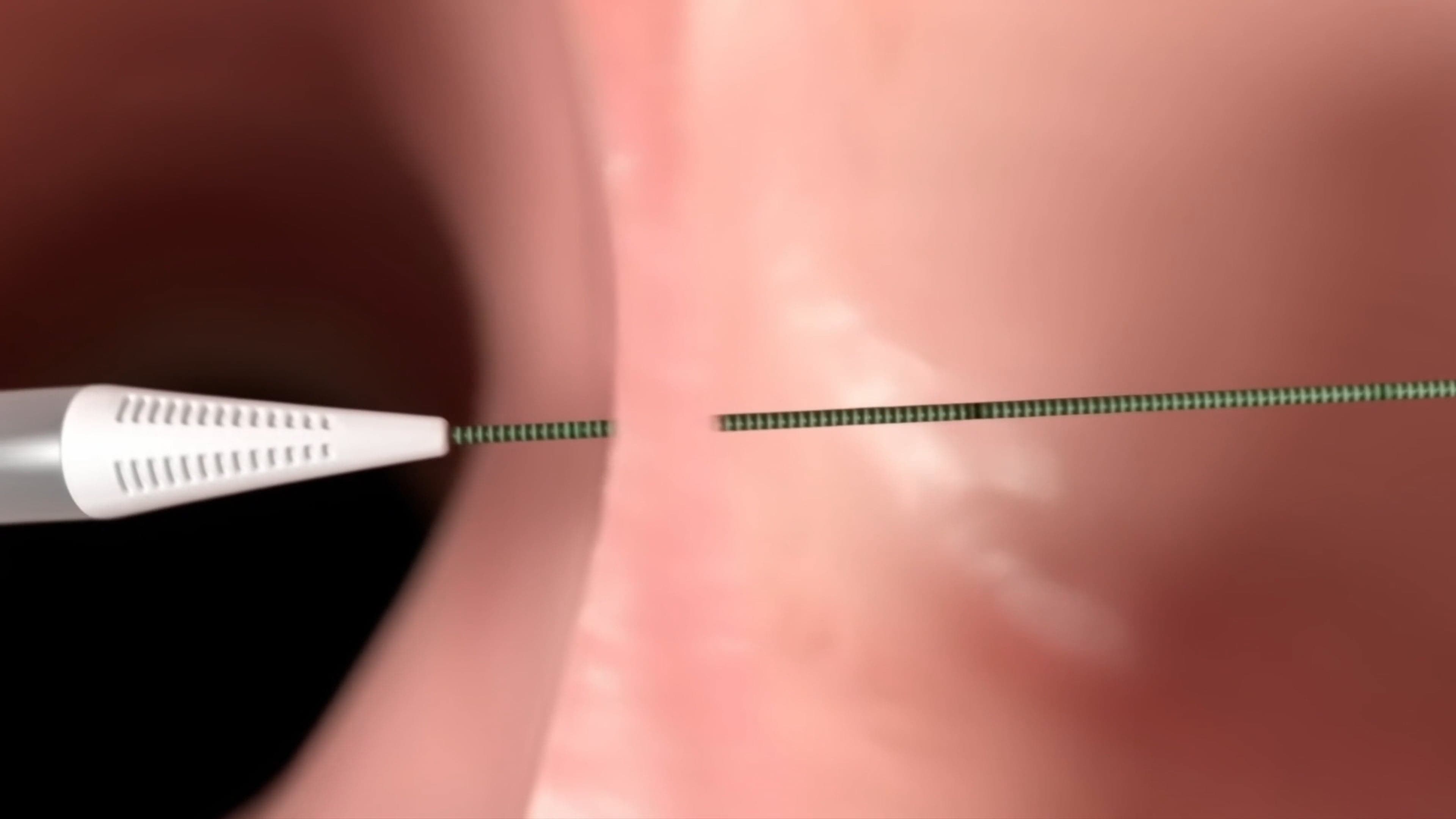
How is the Alleviant shunt created?
Dr. Jacob Kriegel (Cardiothoracic Surgeon and Chief Medical Officer, Alleviant Medical) provides a step-by-step overview of the investigational procedure.






.jpg&w=3840&q=75)

No open heart surgery required.

Ongoing heart failure follow-up evaluations from a team of heart failure specialists over a 5-year period.

The opportunity to help advance new therapies that may improve the future management of heart failure.
CAUTION - Investigational device - Not for sale. Limited by Federal (or United States) law to investigational use. Exclusively for clinical investigation and to be used by qualified investigators only.